ctDNA
Circulating tumour DNA testing in the NHS

Liquid biopsy tests, also known as circulating tumour DNA (ctDNA) tests, sequence fragments of tumour DNA that are released into the blood stream.
In 2022 NHS England (NHSE) commissioned a pilot study to evaluate how ctDNA testing could be integrated into the standard NHS lung cancer pathway and the impact it would have on patients.
The project
The NHS in England is working towards being a global leader to adopt liquid biopsy testing into a national health service, specifically in late-stage lung cancer patients as a priority. While this type of genetic testing is an established technology, there are currently no national healthcare services in the world offering it as mainstream testing.
In 2020, 37,211 people were diagnosed with lung cancer in England with 68% of this population at an advanced stage with limited life expectancy. People with advanced lung cancer have complex care needs and often experience high levels of GP appointments, hospital admissions and extended lengths of stay while awaiting diagnosis and treatment.
However, there are multiple targeted treatments for lung cancer patients, that often increase quality of life of patients. These targeted treatments require genomic testing to identify key genetic drivers of a patient’s cancer. Currently this testing is done through tissue biopsy and results take on average 3-4 weeks to be returned.
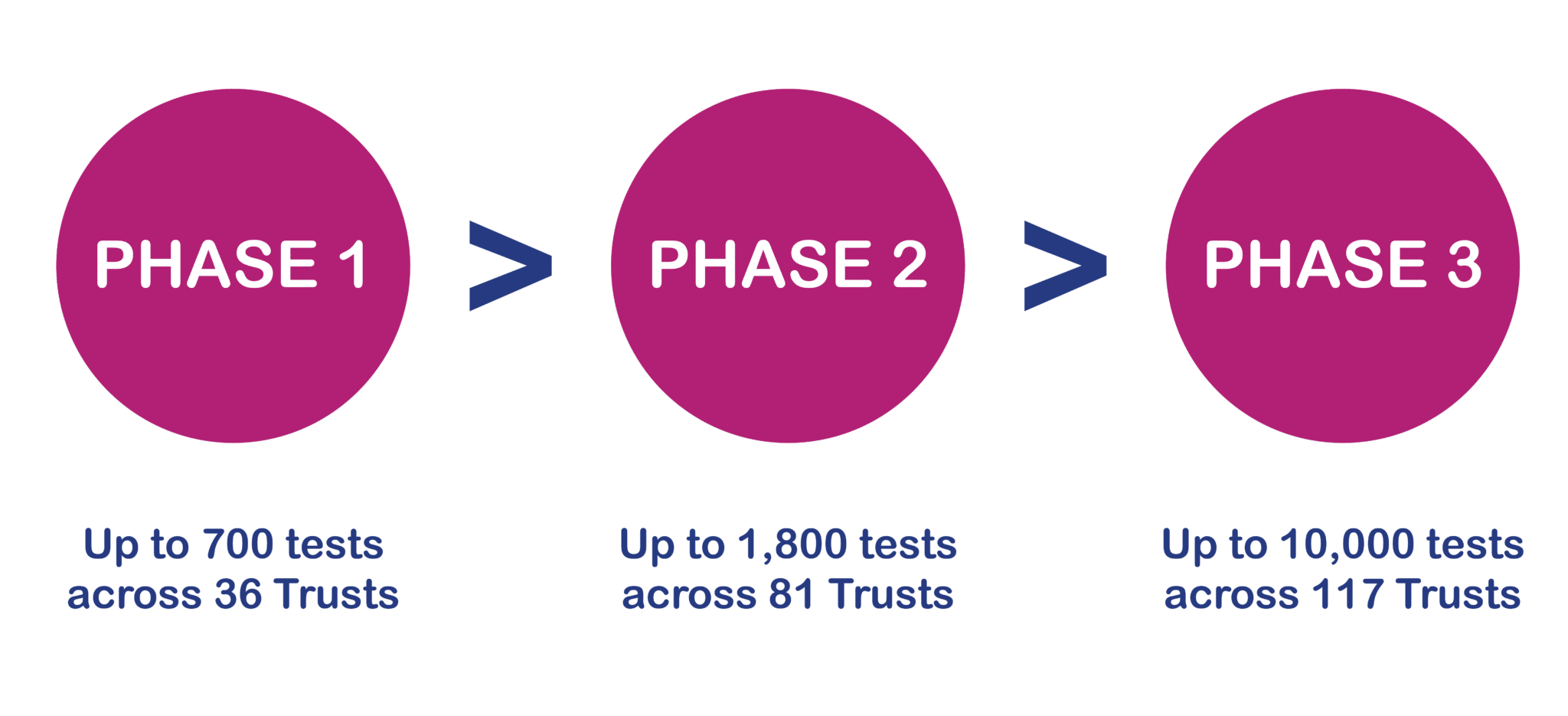
A three-phased pilot study
This NHSE pilot has been run across three phases, led by the North Thames and North-East and Yorkshire GMSA.
In phase 1, each GMSA partnered with a commercial provider, Guardant or Foundation One, to deliver testing using commercially available technology. Phase 2 saw the Royal Marsden NHS laboratory, deliver the testing within an NHS laboratory following a successful technology transfer.
Across the first two phases of the pilot, data was collected to assess the quality of ctDNA testing, turnaround times, outcome of patients with genetic changes and the clinical impact of the test result compared to the current pathway.
As this is an emerging technology, it was also vital to run an economic assessment and evaluation of the new pathway. NHSE commissioned Edge Health to undertake this work to support the ongoing pilot and ensure a full cost/benefit analysis was carried out.
The third phase of the study began in 2024, with a further 10,000 patients being offered the test.